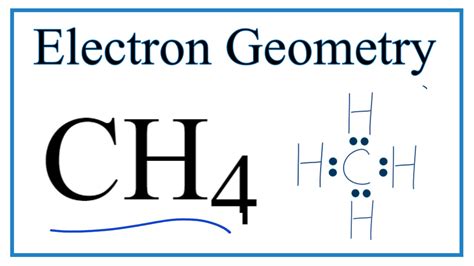
electron geometry ch4|electron geometry chart : Tagatay Methane has 4 regions of electron density around the central carbon atom (4 bonds, no lone pairs). The resulting shape is a regular tetrahedron with H-C-H angles of 109.5°. Click the structures to load . The content on this website (including books, videos and courses) is for informational, educational, and entertainment purposes only. The content provided herein does not constitute professional advice and should not be relied upon as such.
PH0 · number of bonding electron groups in ch4
PH1 · how to determine electron geometry
PH2 · electron geometry vs molecular geometry
PH3 · electron geometry chart
PH4 · electron and molecular geometry table
PH5 · ch4 valence electrons
PH6 · ch4 lewis structure
PH7 · c2h4 electron pair geometry
PH8 · Iba pa
APARTAMENTOS TURÍSTICOS, PONCE DE LEÓN, Deluxe ofrece vistas a la montaña y wifi gratis. Ofrece alojamiento con una ubicación excelente en Ronda, a poca distancia de Iglesia de Santa María la Mayor, Plaza de España y Alameda del Tajo.
electron geometry ch4*******In this video we look at the electron geometry for Methane (CH4). Because the methane molecule has four electron domains (four hydrogen atoms and no lone pairs) the electron .
electron geometry ch4 Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms’ bond formations. The electrons that participate . An explanation of the molecular geometry (and Electron Geometry) for the CH4 (Methane) including a description of the CH4 bond angles.Methane has 4 regions of electron density around the central carbon atom (4 bonds, no lone pairs). The resulting shape is a regular tetrahedron with H-C-H angles of 109.5°. Click the structures to load . A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane). For the CH4 structure use the periodic table to find the total number of valence electrons for the CH4.The CH4 molecule is non-polar due to its symmetrical tetrahedral molecular geometry and the equal sharing of electrons between the carbon and hydrogen atoms. In CH4, the four C-H bonds are arranged .
Lewis Structure for CH4 (Methane) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it .Lewis Structure for CH4 (Methane) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it .
What is the molecular geometry of CH4? The molecular geometry of CH4 is tetrahedral. This means that the four hydrogen atoms are arranged around the central carbon atom .

CH 4 contains 4 bonded and no nonbonded electron domains, giving tetrahedral e-domain and molecular geometries. (AX 3 E 1). The H-C-H bond angles (109.5 degrees) are .electron geometry chartCH 4 contains 4 bonded and no nonbonded electron domains, giving tetrahedral e-domain and molecular geometries. (AX 3 E 1). The H-C-H bond angles (109.5 degrees) are .2. The carbon atom forms two double bonds. Each double bond is a group, so there are two electron groups around the central atom. Like BeH 2, the arrangement that minimizes repulsions places the groups 180° apart. 3. Once again, both groups around the central atom are bonding pairs (BP), so CO 2 is designated as AX 2.
electron geometry ch4 electron geometry chart 1. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is. 2. There are two electron groups around the central atom. .Ammonia, NH3, is a pyramid-shaped molecule, with the hydrogens in an equilateral triangle, the nitrogen above the plane of this triangle, and a H-N-H angle equal to 107°. The geometry of CH4 is that of a tetrahedron, with all H-C-H angles equal to 109.5°. (See also Figure.) Ethane, C2H6, has a geometry related to that of methane.
Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°. In fact, the bond angle is 104.5°. Figure \(\PageIndex{9}\): (a) H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are .
A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale.
CH 4 contains 4 bonded and no nonbonded electron domains, giving tetrahedral e-domain and molecular geometries. (AX 3 E 1).The H-C-H bond angles (109.5 degrees) are those predicted for a perfect tetrahedral geometry. Use your mouse (computer) or fingers (touch screen) to manipulate the Jmol model.
As stated above, molecular geometry and electron-group geometry are the same when there are no lone pairs. The VSEPR notation for these molecules are AX n. "A" represents the central atom and n represents the number of bonds with the central atom. When lone pairs are present, the letter E x is added. The x represents the number . According to the VSEPR (Valence Shell Electrons Pair Repulsion) theory if the electrons count of any molecule is 8 then the molecule adopts tetrahedral geometry. The electrons contribution for C is 4 and four H atoms contribute 1 electron each, so the total electron count will be 8. So, the CH4 lewis structure is tetrahedral.
Seize the Days SM. With over 1,800 properties worldwide, Days Inn helps guests make time with family and friends brighter. Nothing brings us closer like the experience of getting away and creating moments. It’s time we all seize the days!
electron geometry ch4|electron geometry chart